Amylyx (AMLX) Secures the FDA's Approval for ALS Drug
Amylyx Pharmaceuticals, Inc. AMLX has received a major boost as the FDA finally approved its drug for the treatment of adults with amyotrophic lateral sclerosis (ALS) under the brand name of Relyvrio (sodium phenylbutyrate and taurursodiol). Shares were up in after-market trading in response to the news.
The drug, an oral, fixed-dose combination therapy, was previously known as AMX0035 in the United States.
ALS is a progressive and fatal neurodegenerative disorder caused by motor neuron death in the brain and spinal cord. The disease affects approximately 29,000 people in the United States.
The approval of Relyvrio is based on data from the multicenter phase II study, CENTAUR, in 137 participants with ALS encompassing a six-month randomized, placebo-controlled phase and an open-label extension (OLE) long-term follow-up phase. Data showed that the drug significantly slowed the loss of physical function in the randomized, placebo-controlled study.
The FDA’s and Central Nervous System Drugs Advisory Committee voted in a majority (7:2) favoring the drug’s approval for ALS earlier in the month in a surprise move after voting against it in March, questioning the efficacy of the drug.
Thereafter, the FDA extended the target action date from June 2022 to September 2022 to review additional analyses of data from the clinical studies. The FDA determined the submission of data to constitute a major amendment to the NDA, resulting in an extension of the PDUFA goal date.
Nevertheless, the company has finally won approval in the United States despite the controversies regarding the CENTAUR study. Now the commercialization of the drug and uptake thereafter will be in the spotlight. Management expects that specialty pharmacies will be able to start filling prescriptions given by the physicians and ship Relyvrio to ALS patients in the next four to six weeks.
In the year so far, the stock has surged 67.1% against the industry’s 27.5% decline.
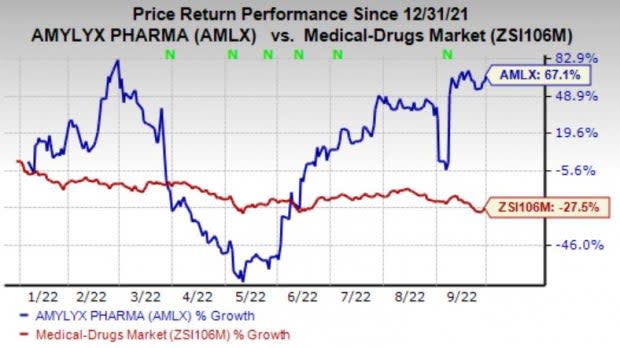
Image Source: Zacks Investment Research
The drug is also under review in the European Union, and a decision is expected in the first half of 2023. AMX0035 has already received marketing authorization in Canada and is being marketed under the trade name Albrioza albeit approved with conditions.
AMX0035 is also being evaluated for the potential treatment of other neurodegenerative diseases.
In May 2022, the FDA approved Radicava ORS (edaravone) oral suspension for the treatment of adults with ALS, marketed by Mitsubishi Tanabe Pharma America, Inc. Radicava ORS is an orally administered version of Radicava, which was originally approved in 2017 as an intravenous (IV) infusion to treat ALS.
Zacks Rank & Stocks to Consider
Amylyx currently carries a Zacks Rank #3 (Hold). Some better-ranked stocks in the healthcare sector are Sanofi SNY, Dynavax DVAX and Bolt Pharmaceuticals BOLT. All three carry a Zacks rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Earnings estimates for Sanofi are up 4 cents each for 2022 and 2023 in the past 30 days. Sanofi surpassed estimates in all of the trailing four quarters, the average being 9.37%.
Loss estimates for BOLT have narrowed to $2.25 from $2.87 in the past 60 days. Earnings surpassed estimates in three of the trailing four quarters and missed the mark in the remaining one, the average beat being 2.39%.
Dynavax’s earnings estimates have increased to $1.73 from $1.14 for 2022 over the past 60 days. Earnings of DVAX surpassed estimates in two of the trailing four quarters and missed the mark in the remaining two, the average beat being 70.57%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Sanofi (SNY) : Free Stock Analysis Report
Dynavax Technologies Corporation (DVAX) : Free Stock Analysis Report
Amylyx Pharmaceuticals, Inc. (AMLX) : Free Stock Analysis Report
Bolt Biotherapeutics, Inc. (BOLT) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

