Altimmune (ALT) Stock Down Despite Positive Obesity Study Data
Altimmune, Inc. ALT announced positive topline results from its interim analysis of the MOMENTUM phase II obesity study on lead pipeline candidate pemvidutide. The company’s stock plunged about 55% on Tuesday following the news. This is likely due to investor dissatisfaction in terms of side effects from treatment with pemvidutide, leading to a high rate of discontinuation of the study.
Pemvidutide is Altimmune’s novel, investigational, peptide-based glucagon-like peptide 1 (GLP-1)/glucagon dual receptor agonist being developed to treat obesity and non-alcoholic steatohepatitis (NASH).
In the past year, shares of Altimmune have plunged 27.9% compared with the industry’s 17.4% decline.
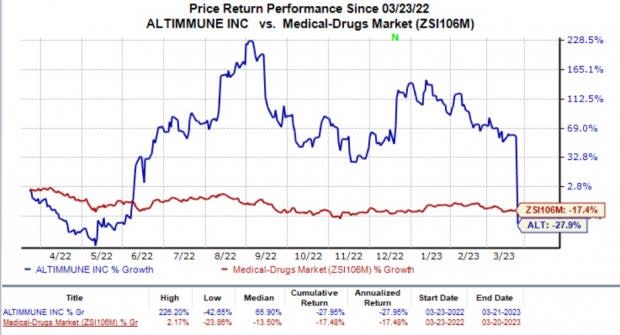
Image Source: Zacks Investment Research
Altimmune’s MOMENTUM phase II study enrolled approximately 320 subjects with obesity or overweight with at least one co-morbidity and without diabetes. The subjects were divided into four cohorts each receiving either 1.2 mg, 1.8 mg, 2.4 mg pemvidutide or placebo administered weekly for 48 weeks in conjunction with diet and exercise. The interim analysis data was from 160 subjects who completed 24 weeks of treatment.
At the end of Week 24, it was observed that the subjects receiving pemvidutide achieved mean weight losses of 7.3%, 9.4% and 10.7% at the 1.2 mg, 1.8 mg, and 2.4 mg doses, respectively, with the placebo group experiencing a mean weight loss of 1.0%. 75% of the subject population comprising subjects with baseline body weight less than or equal to 115 kg, witnessed a mean weight loss of 8.2%, 10.6%, 11.9% and 0.8% at the 1.2 mg, 1.8 mg, 2.4 mg and placebo groups, respectively. 10% or more weight loss was observed in approximately 50% of the subjects while about 20% of subjects achieved 15% or more weight loss at Week 24 at the 1.8 mg and 2.4 mg doses.
A rapid reduction in waist circumference, used to measure visceral fat, and serum lips were also observed, including clinically meaningful reductions in blood pressure, achieved without meaningful increases in heart rate. The safety profile of the candidate was also well-maintained in the study. Adverse events included nausea, vomiting, diarrhea and constipation which were predominantly mild and moderate in severity.
However, a treatment discontinuation rate of 28.2% in subjects receiving the placebo and 24.0% in subjects receiving pemvidutide was observed in the first 16 weeks of treatment. Discontinuation of treatment with pemvidutide was mostly due to the adverse events observed.
If approved, the management believes pemvidutide to be an important treatment option for patients with obesity, especially those who are at risk of cardiovascular disease, liver disease, dyslipidemia and related conditions. The company expects to complete the 48-week MOMENTUM phase II obesity study in the fourth quarter of 2023. Altimmune also plans to initiate a phase II biopsy NASH study in mid-2023.
The company also announced the results of the candidate’s 12-week phase Ib study to treat obesity or overweight and type II diabetes.
In this study, 54 subjects, aged 18-65 years, were again divided into four cohorts receiving either 1.2 mg, 1.8 mg, 2.4 mg pemvidutide or placebo administered weekly for 12 weeks. Average weight losses of 4.4%, 6.1% and 7.7% at the 1.2 mg, 1.8 mg, and 2.4 mg doses of pemvidutide, respectively, were observed over only 12 weeks of treatment.
Compared to the candidate, the placebo group experienced a mean weight gain of 0.8%. In the course of this study, glucose homeostasis was maintained throughout the 12 weeks of treatment, with no significant changes in fasting glucose or HbA1c and no adverse events related to hyperglycemia. No adverse events lead to study discontinuation in this case.
Altimmune, Inc. Price and Consensus
Altimmune, Inc. price-consensus-chart | Altimmune, Inc. Quote
Zacks Rank and Stocks to Consider
Altimmune currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
A few other stocks worth considering are Avalo Therapeutics AVTX, Avita Medical RCEL and EQRx EQRX, each carrying a Zacks Rank #2 (Buy) at present.
In the last 90 days, Avalo’s loss estimates for the year 2023 have narrowed from $3.62 to $2.57 per share. In the past year, shares of Avalo have plunged 72.8%.
AVTX beat estimates in two of the last three reported quarters, missing the mark on one occasion, delivering an average earnings surprise of 37.83%.
In the last 90 days, Avita’s loss estimates for the year 2023 have narrowed from $1.26 to 99 cents per share. In the past year, shares of Avita have plunged 77.2%.
RCEL beat estimates in three of the last four reported quarters, missing the mark on one occasion, delivering an average earnings surprise of 22.16%.
In the last 90 days, EQRx’s loss estimates for the year 2023 have narrowed from 71 cents to 58 cents per share. In the past year, shares of EQRx have fallen 49.2%.
EQRX beat earnings estimates in all the last four reported quarters clocking an average earnings surprise of 34.99%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Altimmune, Inc. (ALT) : Free Stock Analysis Report
Avita Medical Inc. (RCEL) : Free Stock Analysis Report
Avalo Therapeutics, Inc. (AVTX) : Free Stock Analysis Report
EQRx, Inc. (EQRX) : Free Stock Analysis Report

