Reata (RETA) Q3 Earnings Beat, Stock Up on Pipeline Updates
Reata Pharmaceuticals, Inc. RETA reported a loss of $2.16 per share in third-quarter 2022, narrower than the Zacks Consensus Estimate of a loss of $2.35. In the year-ago quarter, the company posted a loss of $1.97.
However, the above loss included stock-based compensation and a non-cash interest expense. Adjusted loss for the quarter was $1.47 per share, wider than the loss of $1.27 per share recorded in the year-ago period.
Total revenues, comprising collaboration revenues, were $0.54 million, significantly down from the year-ago quarter’s revenues of $7.4 million. Revenues missed the Zacks Consensus Estimate of $1 million.
Quarter in Detail
Adjusted research and development expenses were up 8.2% year over year to $36.8 million. Adjusted general and administrative expenses were $19.5 million, up 11.6% from the year-ago period.
The company had cash and cash equivalents and marketable securities of $435.9 million as of Sep 30, 2022, compared with $481.5 million as of Jun 30, 2022. The company expects its cash resources to fund operations through 2024-end.
Pipeline Update
Reata has developed its lead pipeline candidates — bardoxolone methyl (bardoxolone) and omaveloxolone — for rare forms of chronic kidney disease (CKD) and neurological diseases, respectively.
Reata has developed omaveloxolone as a potential treatment for Friedreich’s ataxia (“FA”). A FDA filing for omaveloxolone in FA is currently under review, which was accepted for priority review in May 2022.
Alongside its earnings results, management also announced updates on the NDA filing for omaveloxolone in FA. Management announced that it had recently completed a late-cycle meeting with the FDA to discuss substantive issues identified during the review. The agency is currently reviewing the data submitted by the company per the FDA’s request at the mid-cycle meeting.
Per the FDA, this data submitted by Reata constituted a major amendment to the original NDA and has extended the review period by an additional three months to review the same. A decision from the agency is now expected by February 2023.
While formal minutes for the late-cycle meeting are yet to be received by the company, management confirmed that there is no data request pending from the company’s side though the FDA may request additional data during the review period. The FDA also did not identify any risks related to risk management and also reiterated that no advisory committee meeting will be held for the NDA filing.
Reata shares were up 15.4% on Oct 8 most likely on the positive updates for the omaveloxolone NDA. Shares of Reata have surged 52.8% year to date against the industry’s 21.5% decline.
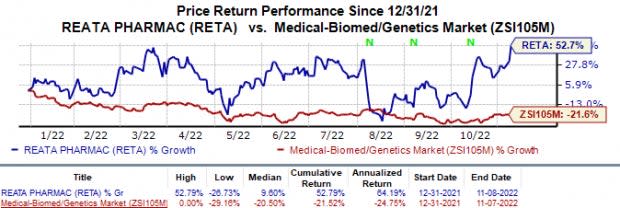
Image Source: Zacks Investment Research
The FDA will also provide an update to omaveloxolone’s post-marketing requirements and comments on the drug’s label by early 2023. The FDA also stated in the late-cycle meeting that a drug-drug interaction trial might be required if the NDA filing is approved.
Reata plans to file a regulatory application seeking approval for omaveloxolone as a treatment for FA patients in Europe by 2022-end. Management has already initiated commercial preparations for omaveloxolone in FA.
Reata is evaluating bardoxolone for treating autosomal dominant polycystic kidney disease (ADPKD) in the ongoing late-stage FALCON study. Based on the result of the data from the FALCON study, Reata will submit a regulatory filing with the FDA. The candidate is also being evaluated in the phase III AYAME study for treating diabetic kidney disease. The AYAME study is being conducted by partner Kyowa Kirin.
Reata Pharmaceuticals, Inc. Price
Reata Pharmaceuticals, Inc. price | Reata Pharmaceuticals, Inc. Quote
Zacks Rank and Stocks to Consider
Currently, Reata has a Zacks Rank #3 (Hold). Some better-ranked stocks in the overall healthcare sector include Angion Biomedica ANGN, Gilead Sciences GILD and Vertex Pharmaceuticals VRTX, each carrying a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Estimates for Angion Biomedica’s 2022 loss per share have narrowed from $1.64 to $1.53 in the past 60 days. Angion’s loss estimates for 2023 have narrowed from $1.54 to $1.43 in the past 60 days. Shares of Angion Biomedica have plunged 70% in the year-to-date period.
Earnings of Angion Biomedica beat estimates in three of the last four quarters and missed the mark on one occasion, the average surprise being 62.85%. In the last reported quarter, Angion Biomedica delivered an earnings surprise of 34.78%.
Gilead’s stock has risen 12.2% this year so far. While Gilead’s earnings estimates for 2022 have risen from $6.62 to $7.02 per share in the past 60 days, estimates for 2023 have increased from $6.32 to $6.79 per share during the same period.
Gilead beat earnings estimates in three of the last four quarters and missed the mark on one occasion, the average surprise being 0.36%. In the last reported quarter, Gilead delivered an earnings surprise of 31.94%.
Vertex’s stock has risen 39.6% this year so far. While Vertex’s earnings estimates for 2022 have risen from $14.21 to $14.61 per share in the past 60 days, estimates for 2023 have increased from $15.12 to $15.60 per share during the same period.
Vertex beat earnings estimates in three of the last four quarters and missed the mark on one occasion, the average surprise being 3.16%. In the last reported quarter, Vertex reported an earnings surprise of 8.67%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Gilead Sciences, Inc. (GILD) : Free Stock Analysis Report
Vertex Pharmaceuticals Incorporated (VRTX) : Free Stock Analysis Report
Angion Biomedica Corp. (ANGN) : Free Stock Analysis Report
Reata Pharmaceuticals, Inc. (RETA) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

