Pharma Stock Roundup: NVS to Spin Off Sandoz; JNJ, MRK, ABBV's FDA Updates
This week, Novartis NVS announced the planned spin-off of its generics division, Sandoz, to create a leading European generics company. The FDA granted approval to J&J JNJ and AbbVie’s ABBV cancer drug Imbruvica for a pediatric indication and gave Fast Track designation to Merck’s MRK investigational anticoagulant therapy, MK-2060. Pfizer PFE filed an application seeking emergency authorization from the FDA for its Omicron BA.4/BA.5-adapted bivalent COVID-19 booster vaccine.
Recap of the Week’s Most Important Stories
Novartis to Spin Off Sandoz Into a New Co.: Novartis announced that it plans to divest its generics and biosimilars division, Sandoz, by way of a 100% spin-off into a new publicly traded company. The new Sandoz will be headquartered in Switzerland. The transaction is expected to be tax-neutral for Novartis and is likely to be completed by the second half of 2023, though Novartis has not received any formal offers for the unit. In October 2021, Novartis began a strategic review of the Sandoz Division. After examining all options, management has decided that a company spin-off will be in the shareholders' best interest. The Sandoz spin-off will leave behind Novartis’ Innovative Medicines unit — its core drug development business.
FDA Approves J&J/AbbVie’s Imbruvica for Pediatric cGVHD: The FDA approved J&J and AbbVie’s cancer blockbuster medicine, Imbruvica, to treat pediatric patients with chronic graft versus host disease (cGVHD). The approval makes Imbruvica the first and currently the only approved treatment for children under 12 years of age suffering from cGVHD. The FDA decision is based on data from the phase I/II iMAGINE study.
AbbVie has developed Imbruvica in collaboration with J&J. Per the terms of the agreement, AbbVie and J&J jointly market Imbruvica in the United States, while J&J holds exclusive rights for marketing the drug outside the country.
The European Commission granted conditional marketing authorization (CMA) to J&J’s off-the-shelf bispecific antibody, Tecvayli (teclistamab) as monotherapy for treating patients with relapsed and refractory multiple myeloma. This marks the first approval for Tecvayli in any country. Tecvayli’s approval was based on data from the phase I/II MajesTEC-1 study, which showed that treatment with teclistamab resulted in deep and durable responses.
Pfizer Seeks FDA Authorization for Omicron Variant Booster: Pfizer/BioNTech completed filing an application seeking Emergency Use Authorization (EUA) for a 30-µg booster dose of their bivalent Omicron BA.4/BA.5-adapted bivalent COVID-19 vaccine for individuals 12 years of age and older. The filing includes data from studies on their bivalent Omicron BA.1-adapted vaccine as well as pre-clinical and manufacturing data from their Omicron BA.4/BA.5-adapted bivalent COVID-19 vaccine. Pfizer/BioNTech have already scaled production of the Omicron BA.4/BA.5-adapted bivalent boosters and will be ready to ship doses as soon as the FDA grants EUA.
The companies have also initiated the filing of a conditional marketing authorization application with the European Medicines Agency (EMA) for the Omicron BA.4/BA.5-adapted bivalent vaccine, which is expected to be completed in the coming weeks.
Pfizer/BioNTech announced new data from a phase II/III study evaluating a three 3-µg dose series of its vaccine in children six months through four years of age. The FDA granted emergency authorization for use of the vaccine in this age group in June based on preliminary data based on 10 symptomatic COVID-19 cases from the same study. The updated data based on 34 cases showed 73.2% vaccine efficacy among children in this age group. Most cases were caused by the Omicron BA.2 variant. Pfizer/BioNTech are preparing an EUA application for an Omicron BA.4/BA.5-adapted bivalent vaccine in children six months through 11 years of age.
Pfizer’s bivalent respiratory syncytial virus (RSV) vaccine candidate, or RSVpreF, was found to be effective in a phase III study on the candidate to prevent lower respiratory tract illness caused by RSV in individuals aged 60 years and above. Data from the interim analysis of the RENOIR study demonstrated vaccine efficacy of 66.7% in participants with two or more RSV-associated symptoms. However, the vaccine efficacy was 85.7% in participants with more severe disease having three or more symptoms.
FDA’s Fast Track Tag for Merck’s Anticoagulant Therapy: The FDA granted Fast Track designation to Merck’s pipeline candidate, MK-2060, an anticoagulant therapy for the reduction in risk of major thrombotic cardiovascular events in patients with end-stage renal disease (ESRD). A phase II study is ongoing on MK-2060 in people with ESRD receiving hemodialysis. In order to develop effective and safer anticoagulants, Factor XI, a blood protein, is being actively pursued as a drug target. MK-2060 has been designed to inhibit Factor XI’s ability to activate downstream proteins involved in the blood coagulation cascade.
The NYSE ARCA Pharmaceutical Index declined 0.19% in the last five trading sessions.
Large Cap Pharmaceuticals Industry 5YR % Return
Large Cap Pharmaceuticals Industry 5YR % Return
Here’s how the eight major stocks performed in the last five trading sessions.
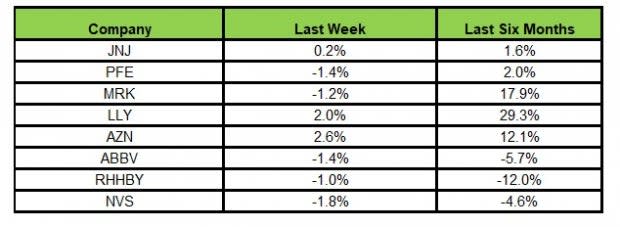
Image Source: Zacks Investment Research
In the last five trading sessions, AstraZeneca was the highest gainer (2.6%) while Novartis declined the most (1.8%).
In the past six months, Lilly has gained the highest (29.3%) while Roche declined the most (12.0%).
(See the last pharma stock roundup here: End of SNY’s Amcenestrant Development, FDA Updates for AZN, GSK)
What's Next in the Pharma World?
Watch out for regular pipeline and regulatory updates next week.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Novartis AG (NVS) : Free Stock Analysis Report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
AbbVie Inc. (ABBV) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

