Pharma Stock Roundup: JNJ, MRK M&A Deals, SNY, RHHBY Drugs' Priority Review & More
This week, J&J JNJ and Merck MRK announced proposed acquisitions of private biotech companies. The FDA granted priority review designations to Sanofi's SNY and Roche’s RHHBY regulatory applications seeking expanded use of Sanofi’s multiple myeloma drug, Sarclisa and Roche’s breast cancer candidate, inavolisib. AstraZeneca AZN announced overall survival (“OS”) data from a phase III lung cancer study on it and partner Daiichi Sankyo’s candidate, datopotamab deruxtecan (Dato-DXd).
Recap of the Week’s Most Important Stories
J&J Buys Rights to Novel Atopic Dermatitis Candidate: J&J announced a definitive agreement with Numab Therapeutics to acquire global rights to NM26, a phase II-ready bispecific antibody targeting two clinically proven disease-driving pathways in atopic dermatitis (AD), IL-4R alpha subunit and IL-31. J&J will acquire the rights to NM26 from Numab’s shareholders through its wholly-owned subsidiary called Yellow Jersey Therapeutics. Numab Therapeutics will spin off Yellow Jersey Therapeutics to its shareholders. J&J will buy Yellow Jersey Therapeutics, which has the rights to NM26, from Numab’s shareholders for $1.25 billion in cash. The transaction is expected to be closed in the second half of 2024. J&J believes that NM26 has the potential to transform the standard of care for AD.
J&J’s pipeline candidate seltorexant met all primary and secondary endpoints in a pivotal phase III study in patients with major depressive disorder (MDD) with insomnia symptoms. Top-line data from the study showed that seltorexant led to statistically significant and clinically meaningful improvement in depressive symptoms and sleep disturbance outcomes in patients who had a prior inadequate response to SSRI/SNRI antidepressants alone. The improvement in depressive symptoms was measured on the Montgomery-Asberg Depression Rating Scale. At present, no therapies are approved to treat MDD with insomnia symptoms.
Merck to Buy Ophthalmology Biotech: Merck announced a definitive agreement to acquire London-based private biotech Eyebiotech Limited (“EyeBio”), which will expand its presence in the growing ophthalmology market. The acquisition will add EyeBio’s lead pipeline candidate, Restoret, a novel Wnt agonist antibody, being developed for treating retinal diseases like diabetic macular edema (DME) and neovascular age-related macular degeneration in a phase Ib/IIa study called AMARONE. In February, EyeBio announced first-in-human data from the study. Proof of concept was established in the study with strong visual and anatomic outcomes. The data, for the first time, showed that activating the Wnt pathway in the retina results in a reduction in vascular leakage. This was the first clinical evidence that validated the Wnt pathway in the eye.
Based on the positive data, EyeBio plans to advance Restoret to a pivotal phase IIb/III study for DME in the second half of 2024. Merck will make a $1.3 billion upfront payment for acquiring EyeBio through a subsidiary. EyeBio will also be eligible to receive up to $1.7 billion in future milestone payments, bringing the potential value of the transaction to $3 billion.
FDA Delays Decision on Sanofi’s Dupixent sBLA for COPD: The FDA has extended its decision on a supplemental biologics license application (sBLA) seeking approval of Dupixent for the treatment of controlled chronic obstructive pulmonary disease (COPD) in patients with type II inflammation. The PDUFA date has been pushed to Sep 27 from Jun 27. If approved by the FDA, COPD will be the sixth indication for Dupixent. The sBLA was based on data from two studies called NOTUS and BOREAS. The FDA had asked for additional efficacy analyses on the efficacy of Dupixent from these two studies. Sanofi submitted these additional analyses in May, which the FDA said constituted a major amendment to the sBLA. This led to a delay in the PDUFA date.
The European Medicines Agency’s Committee for Medicinal Products for Human Use also adopted a positive opinion recommending the approval of Dupixent for the COPD indication.
AstraZeneca’s Overall Survival Data From Lung Cancer Study: AstraZeneca announced OS data from TROPION-Lung01 phase III evaluating it and partner Daiichi Sankyo’s TROP2-directed DXd antibody-drug conjugate, Dato-DXd, for locally advanced or metastatic non-small cell lung cancer (NSCLC) treated with at least one prior line of therapy.
The data showed that in the overall trial population, though OS results numerically favored Dato-DXd, it failed to reach statistical significance. In a subgroup of patients with nonsquamous NSCLC, a clinically meaningful improvement in OS was seen compared to docetaxel, the current standard-of-care chemotherapy. The study had previously met the dual primary endpoint of progression-free survival (PFS) in the overall trial population. Also, a clinically meaningful PFS benefit was seen in patients with nonsquamous NSCLC.
AstraZeneca and Daiichi Sankyo have already filed a biologics license application (BLA) seeking approval of Dato-DXd for advanced nonsquamous NSCLC based on PFS data from the TROPION-Lung01 study in the United States. The FDA’s decision on the BLA is expected in the fourth quarter of 2024. Regulatory applications are also under review in other countries, including the EU.
FDA’s Priority Review Tag: The FDA accepted and granted priority review status to a sBLA seeking expanded use of Sarclisa (isatuximab) for newly diagnosed multiple myeloma (NDMM) patients who are not eligible for transplant. The sBLA seeks approval for Sarclisa in combination with Velcade, Revlimid (lenalidomide) and dexamethasone (VRd) for the treatment of patients with NDMM who are transplant-ineligible. The FDA’s decision on the sBLA is expected on Sep 27, 2024. Sarclisa is presently approved as a combination regimen for previously treated patients with relapsed refractory MM.
The FDA also granted priority review status to a new drug application seeking approval of Roche’s pipeline candidate inavolisib for treating breast cancer in the United States. The NDA seeks approval of inavolisib in combination with Ibrance (palbociclib) and Faslodex (fulvestrant) to treat adult patients with PIK3CA-mutated, hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER)-negative, locally advanced or metastatic breast cancer following recurrence on or within 12 months of completing adjuvant endocrine treatment. The priority review is based on positive data from the phase III INAVO120 study. The FDA’s decision on the NDA is expected on Nov 27, 2024.
The NYSE ARCA Pharmaceutical Index declined 1.5% in the last five trading sessions.
Large Cap Pharmaceuticals Industry 5YR % Return
Large Cap Pharmaceuticals Industry 5YR % Return
Here’s how the eight major stocks performed in the last five trading sessions.
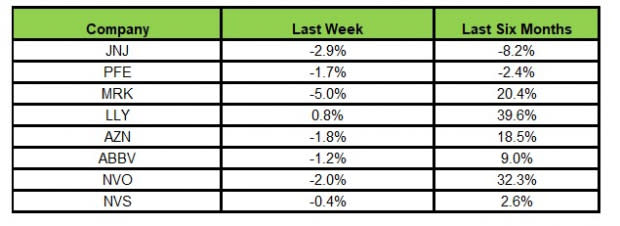
Image Source: Zacks Investment Research
In the last five trading sessions, Lilly rose the most (0.8%), while Merck declined the most (5.0%).
In the past six months, Lilly has risen the most (39.6%), while J&J has declined the most (8.2%).
(See the last pharma stock roundup here: AZN’s 2030 Sales Target, PFE’s New Cost Cut Plan & More)
What's Next in the Pharma World?
Watch this space for regular pipeline and regulatory updates next week.
J&J, Merck, Sanofi and AstraZeneca have a Zacks Rank #3 (Hold) each, while Roche has a Zacks Rank of 4 (Sell).
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Sanofi (SNY) : Free Stock Analysis Report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report

