Pharma Stock Roundup: FDA's CRL to MRK & ABBV, Phase III Study Failures for NVO, AZN
This week, the FDA rejected Merck's MRK and AbbVie’s ABBV regulatory filings seeking approvals for pipeline candidates, patritumab deruxtecan and ABBV-951, respectively. AstraZeneca AZN announced the failure of an early-stage lung cancer study on its PD-L1 inhibitor, Imfinzi. Novo Nordisk’s NVO acquired candidate ocedurenone failed in a phase III hypertension study.
Recap of the Week’s Most Important Stories
FDA Rejects Merck’s and AbbVie’s Pipeline Candidates: The FDA issued a complete response letter (“CRL”) to Merck and its partner Daiichi Sankyo’s biologics license application (BLA) seeking accelerated approval for patritumab deruxtecan for previously-treated EGFR-mutated non-small cell lung cancer (NSCLC). The CRL was based on observations made after the inspection of a third-party manufacturing facility. The FDA has not requested any additional efficacy/safety studies, nor has it identified any issues related to the safety and efficacy of the candidate.
Merck said it will work closely with the FDA and the third-party manufacturer to resolve the issue. It acquired global co-development and co-commercialization rights to patritumab deruxtecan/MK-1022 and two other ADCs, raludotatug deruxtecan/MK-5909 and ifinatamab deruxtecan/MK-2400, from Daiichi Sankyo in October last year.
The FDA also issued a CRL to AbbVie’s new drug application seeking approval for pipeline candidate ABBV-951 for the treatment of motor fluctuations in patients with advanced Parkinson's disease. The latest CRL was based on some observations on inspection of one of AbbVie's third-party manufacturing facilities listed in the NDA. The inspection did not involve ABBV-951 or any other AbbVie drug. The FDA has not requested any additional efficacy/safety studies, nor has it identified any issues related to the safety, efficacy or labeling of ABBV-951, including the device.
This was the second CRL issued for ABBV-951 by the FDA. AbbVie received the first CRL in March last year.
AbbVie Acquires Private IBD Disease Drugmaker: AbbVie acquired Celsius Therapeutics, a private biotech making novel therapies for treating inflammatory diseases (“IBD”). The acquisition will add Celsius Therapeutics’ lead pipeline candidate, CEL383, a potential first-in-class anti-TREM1 antibody that has completed phase I study in IBD conditions. AbbVie has acquired all outstanding equity of Celsius Therapeutics for $250 million in cash, subject to certain customary adjustments.
The FDA granted accelerated approval for the expanded use of AbbVie’s lymphoma drug Epkinly (epcoritamab). The FDA has now approved the drug for treating relapsed or refractory (R/R) follicular lymphoma (FL) after two or more therapies. Epkinly was approved for relapsed-refractory third-line diffuse large B-cell lymphoma (DLBCL) in 2023. The latest approval is based on data from the FL cohort of the phase I/II EPCORE NHL study.
CDC Recommends Merck’s New Pneumococcal Vaccine Capvaxive: The U.S. Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) unanimously recommended Merck’s newly approved 21-valent pneumococcal conjugate vaccine Capvaxive. The FDA approved Capvaxive earlier this month for the prevention of invasive pneumococcal disease and pneumococcal pneumonia in adults. The ACIP recommended Capvaxive for all adults 65 years of age and older and adults aged 19 to 64 with certain risk conditions and for those over 65 previously vaccinated with other pneumococcal vaccines.
Capvaxive targets serotypes that account for approximately 84% of all invasive pneumococcal disease in older adults (50 years and older) in the United States, including eight serotypes not covered by currently licensed vaccines.
AstraZeneca’s Early-Stage NSCLC Study Fails: AstraZeneca’s phase III study evaluating its drug Imfinzi for an early-stage non-small cell lung cancer (NSCLC) indication failed to achieve statistical significance for the primary endpoint of disease-free survival versus placebo. The study called ADJUVANT BR.31 evaluated Imfinzi versus placebo in early-stage (Stage IB-IIIA) NSCLC after complete tumor resection in patients whose tumors express PD-L1 on 25% or more tumor cells. Imfinzi is presently approved for treating unresectable Stage III NSCLC and is being studied for several other early-stage lung cancer settings.
AstraZeneca also announced positive data from the phase III NIAGARA study evaluating Imfinzi plus chemotherapy for muscle-invasive bladder cancer. In the study, Imfinzi, in combination with chemotherapy, demonstrated a statistically significant and clinically meaningful improvement in the primary endpoint of event-free survival and the key secondary endpoint of overall survival versus neoadjuvant chemotherapy. If approved, this will be the first approval for Imfinzi in the bladder cancer indication.
Novo Nordisk Ends Hypertension Study on Ocedurenone: Novo Nordisk’s phase III study called CLARION-CKD, evaluating ocedurenone to treat uncontrolled hypertension and advanced chronic kidney disease (CKD), failed to achieve its primary endpoint. Novo Nordisk has decided to stop the study and will recognize an impairment cost of around DKK 5.7 billion ($816 million) to account for ocedurenone’s failure in the second-quarter results.
Based on interim analysis, an independent data monitoring determined that the study met the prespecified futility criteria, which means it did not meet its primary endpoint of reducing systolic blood pressure from baseline. This conclusion triggered Novo Nordisk's decision to halt the CLARION-CKD study.
The impairment charge will hurt Novo Nordisk’s operating profit growth by around 6 percentage points at constant exchange rates in 2024. The drug was acquired by Novo Nordisk from KBP Biosciences in 2023. The discontinued CLARION-CKD was conducted by KBP Biosciences.
Novo Nordisk announced plans to invest $4.1 billion (approx DKK 27 billion) to build a second fill and finishing manufacturing facility in North Carolina. Novo Nordisk expects to manufacture injectable treatments for obesity and other chronic diseases at the expanded facility. Novo also said that it will significantly boost its production investments to approximately $6.8 billion (DKK 45 billion) in 2024, nearly doubling the previous year's investment of $3.9 billion (DKK 26 billion), excluding acquisitions, to enhance supply capabilities.
The NYSE ARCA Pharmaceutical Index rose 0.8% in the last five trading sessions.
Large Cap Pharmaceuticals Industry 5YR % Return
Large Cap Pharmaceuticals Industry 5YR % Return
Here’s how the eight major stocks performed in the last five trading sessions.
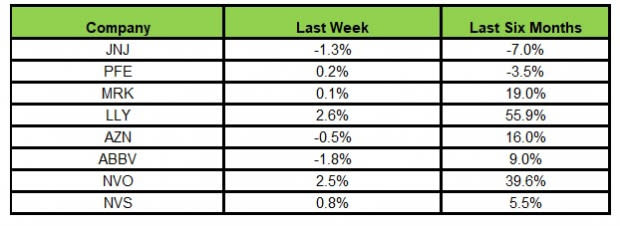
Image Source: Zacks Investment Research
In the last five trading sessions, Lilly rose the most (2.6%), while AbbVie declined the most (1.8%).
In the past six months, Lilly has risen the most (55.9%), while J&J has declined the most (7.0%).
(See the last pharma stock roundup here: FDA Nod for MRK’s New Jab & Expanded Use of ABBV & AZN Drugs)
What's Next in the Pharma World?
Watch this space for regular pipeline and regulatory updates next week.
Merck, Novo Nordisk, AbbVie and AstraZeneca have a Zacks Rank #3 (Hold) each. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Novo Nordisk A/S (NVO): Free Stock Analysis Report
Merck & Co., Inc. (MRK): Free Stock Analysis Report
AbbVie Inc. (ABBV): Free Stock Analysis Report

