Pharma Stock Roundup: EU Nod to AZN, SNY, JNJ Drugs, Glaxo's New COVID-19 Drug Data
This week, the European Commission approved AstraZeneca’s AZN Koselugo to treat children with rare disease, neurofibromatosis type 1 (NF1) and allowed expanded use of J&J’s JNJ Darzalex Faspro for two new indications and Sanofi’s SNY Aubagio for pediatric patients. Glaxo GSK/Vir Biotech’s new data confirms that their COVID-19 treatment, sotrovimab can cut hospitalization and risk of death.
Recap of the Week’s Most Important Stories
EU Approves AstraZeneca’s Koselugo for Rare Disease NF1 in Kids: The European Commission granted conditional approval to AstraZeneca’s Koselugo (selumetinib) for the treatment of pediatric patients with NF1 related plexiform neurofibromas (PN), a rare and debilitating genetic condition. Koselugo is the first medicine approved in Europe for NF1 related PN. The approval was based on data from the SPRINT Stratum 1 phase II study, which demonstrated that Koselugo shrank tumor size in some children, reduced pain and improved quality of life. Koselugo was approved by the FDA in April 2020.
European Approval for Two New Indications of J&J’s Darzalex Faspro: The European Commission granted marketing approval for expanded use of Darzalex Faspro for two new indications in Europe. Darzalex Faspro is the subcutaneous formulation of J&J’s blockbuster multiple myeloma drug, Darzalex.
The first indication is for expanded use of Darzalex SC in combination with cyclophosphamide, bortezomib and dexamethasone for the treatment of newly diagnosed systemic light chain (AL) amyloidosis. The second indication is for combination use with pomalidomide and dexamethasone (D-Pd) to treat adult patients with pre-treated multiple myeloma (MM).
J&J submitted a new drug application to the FDA, seeking approval of its oral factor Xa inhibitor Xarelto to treat and prevent venous thromboembolism (VTE) or blood clots in children. The NDA seeks approval for two indications, for the treatment of VTE and prevention of VTE recurrence in children and adolescents. The NDA application is based on data from two phase III studies of Xarelto in pediatric populations, EINSTEIN-Jr and UNIVERSE. Xarelto is already approved in EU, U.K. Canada and Switzerland for the same indications in children. J&J markets Xarelto in partnership with Bayer to treat and prevent blood clots in adults.
Glaxo and Vir Biotech’s Final Data from COMET Study on Sotrovimab: Glaxo and partner Vir Biotech announced final day 29 data from the COMET study, which confirmed that their monoclonal antibody treatment, sotrovimab, significantly reduces hospitalization and risk of death in adults with mild-to-moderate COVID-19 who are at high risk of progression to severe disease. Data demonstrated a 79% reduction in hospitalization for more than 24 hours or death compared with placebo after 29 days.
Additionally, the company announced that the U.S. National Institutes of Health (NIH) updated its COVID-19 treatment guidelines to recommend use of sotrovimab. The companies also said they have approximately 450,000 doses of sotrovimab available for supply in the United States. Sotrovimab was granted emergency approval to treat high-risk COVID-19 by the FDA in May 2021. The companies plan to submit a formal biologics license application (BLA) seeking approval of sotrovimab in the second half of 2021.
Sanofi/Translate Bio Begin Study on mRNA Flu Vaccine Candidate: Sanofi and partner Translate Bio initiated a phase I study on their mRNA-based vaccine candidate against seasonal influenza. Interim data from the study is expected to be presented by the end of 2021. The study will evaluate two formulations of the influenza mRNA vaccine (MRT5400 and MRT5401) in the phase I study. The phase I study was initiated following promising safety and immunogenicity results in preclinical studies.
Meanwhile, the European Commission approved Sanofi’s multiple sclerosis drug, Aubagio for first-line treatment of children and adolescents with relapsing-remitting multiple sclerosis.
Merck’s Keytruda Study in First-Line Cervical Cancer Meets Primary Endpoint: Merck’s MRK phase III study evaluating its PD-L1 inhibitor, Keytruda as a first line treatment of patients with persistent, recurrent or metastatic cervical cancer, met its primary endpoints of overall survival (OS) and progression-free survival (PFS). Data from the phase III KEYNOTE-826 study showed that Keytruda plus platinum-based chemotherapy with or without bevacizumab significantly improved OS and PFS compared to platinum-based chemotherapy with or without bevacizumab alone, regardless of PD-L1 status. Keytruda is presently approved as a second-line treatment of patients with recurrent or metastatic cervical cancer.
Pfizer Begins Pivotal Study of Cancer Drugs in Advanced Prostate Cancer: Pfizer PFE announced dosing of the first participant in a pivotal phase III study evaluating a combination of its cancer drugs, PARP inhibitor, Talzenna (talazoparib) and androgen receptor inhibitor, Xtandi (enzalutamide) in metastatic castration-sensitive prostate cancer (mCSPC). The study will enroll approximately 550 men with DDR-deficient mCSPC across 28 countries. The first patient was dosed at a site in California. The primary endpoint of the study is radiographic progression-free survival (rPFS) while the secondary endpoint will be OS. While Talzenna is approved for a metastatic breast cancer indication, Xtandi is approved to treat castration-resistant prostate cancer (CRPC) and mCSPC.
The NYSE ARCA Pharmaceutical Index rose 0.2% in the last five trading sessions.
Large Cap Pharmaceuticals Industry 5YR % Return
Large Cap Pharmaceuticals Industry 5YR % Return
Here’s how the eight major stocks performed in the last five trading sessions.
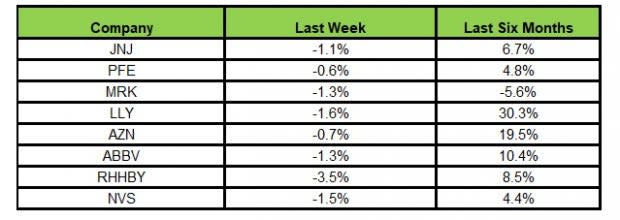
Image Source: Zacks Investment Research
In the last five trading sessions, all the stock were in the red with Roche recording the maximum decline (3.5%).
In the past six months, Lilly LLY has recorded the maximum gain (30.3%) while Merck declined the most (5.6%)
(See the last pharma stock roundup here: AZN COVID-19 Antibody Cocktail Failure, GSK New Cancer Deal)
What's Next in the Pharma World?
Watch out for regular pipeline and regulatory updates next week.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 77 billion devices by 2025, creating a $1.3 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 4 tickers for taking advantage of it. If you don't buy now, you may kick yourself in 2022.
Click here for the 4 trades >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Sanofi (SNY) : Free Stock Analysis Report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
GlaxoSmithKline plc (GSK) : Free Stock Analysis Report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

