Oxford coronavirus vaccine: Does it work? And what happens next?


Oxford scientists believe they have made a breakthrough in their quest for a Covid-19 vaccine. Here's what you need to know.
Does it work?
The clinical trials have so far been successful, the Telegraph understands.
Researchers have shown that antibodies produced against sections of a genetic material called spike protein, usually found on the surface of the coronavirus, after infection are able to kill the virus when tested in the laboratory.
They want the human body to recognise and develop antibodies to the protein that would stop the virus from entering human cells.
Trials suggest that not only is this happening but also that the body is developing a crucial T-cell response as well, which is deemed crucial in the defence against coronavirus.
When will it be available?
AstraZeneca is on track to begin rolling out up to two billion doses of a coronavirus vaccine in September, if ongoing trials continue to prove successful.
Chief executive Pascal Soriot revealed last month that they were already starting to manufacture the vaccine.
Prof Adrian Hill, director of the Jenner Institute at the University of Oxford, has said the "best scenario" would see results from clinical trials in August and September and deliveries from October.
In May, Alok Sharma, the Business Secretary, said Britain would be the first to get the vaccine and announced an extra £84 million in funding to accelerate research.

What is the next step?
As the level of coronavirus in the UK subsided, scientists began trialling the drug in hospitals, where it was likely to be more prevalent.
They have also enrolled 5,000 volunteers in Brazil and others in South Africa.
Developers are expected to report their Phase I study results - which would show whether it is safe and whether or not it induces an immune response - within the next fortnight.
The global vaccine race: How other countries are developing a jab
Worldwide there are some 218 vaccines in development, according to the London School of Hygiene and Tropical Medicine's vaccine tracker.
Of these, some 29 are in human trials - though experts caution that there is no guarantee any of these will be effective. Or it could be that early versions of immunisations offer only partial protection from disease, reducing Covid-19 from a deadly illness to something comparable to a common cold.
The Oxford vaccine is one of the most advanced, described by the World Health Organisation’s chief scientists as a “leading candidate” partly because it has recruited a large number of patients with trials underway in Brazil and South Africa.
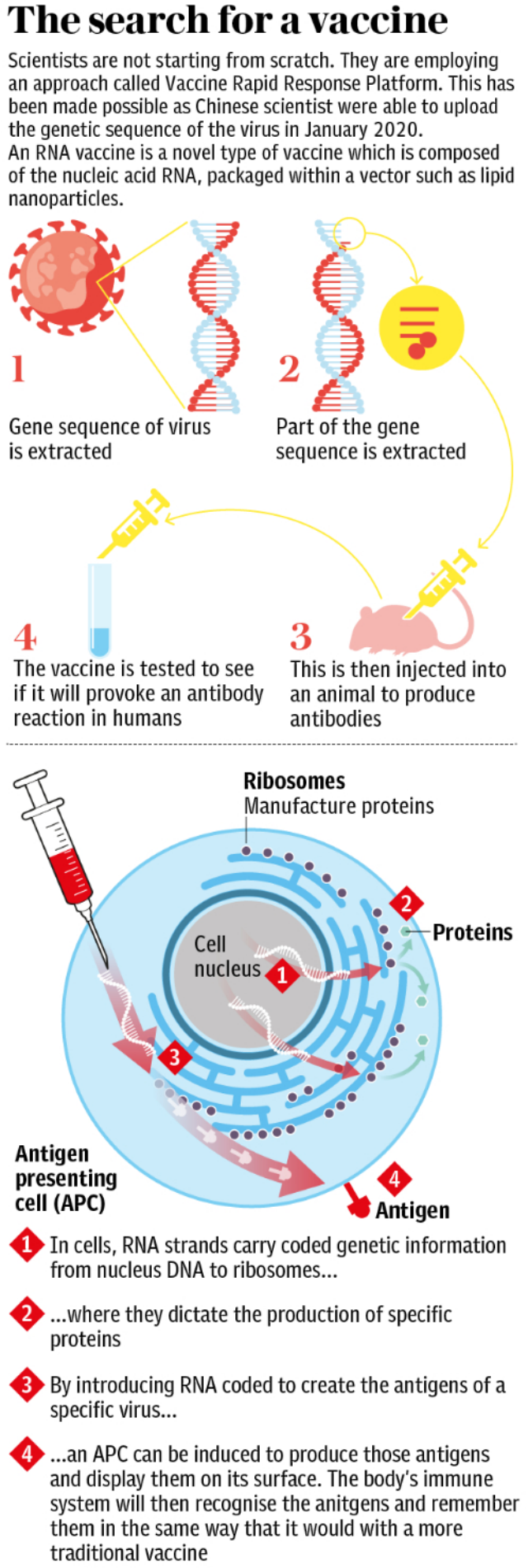
But it is not the only vaccine shown to activate a dual response in trial participants.
This week the US biotech company, Moderna, published data from a phase one trial in 45 participations in the New England Journal of Medicine, which showed that its RNA vaccine triggered both neutralising antibodies and T cells.
This is the same technology as a team at Imperial College London are using, and experts say that the presence of T cells is significant as this vaccine platform has not previously been used in a fully-licensed immunisation.
Meanwhile Inovio, another American biotech, has said that their DNA based vaccine triggered an antibody and T-cell response in 34 of 36 patients in a recent trial - though they are yet to publish data to back up their press release.
And in China CanSino Biologics has shown that its adenovirus-based candidate elicits antibodies and T cells some 14 days post-vaccination. This immunisation has been licensed for partial use within the Chinese military.
Are these other vaccines effective?
Prof Jonathan Ball, a virologist at the University of Nottingham, said that other vaccine technologies are not expected to activate both aspects of our immune system.
Those such as the Chinese Sinovac candidate, which uses an inactivated or dead version of Sars-Cov-2, are unlikely to trigger the production of T cells - at least 17 other vaccine candidates fall into this category.
“A dead virus particle is not going to get inside the human cell in great quantities, so you’re only going to elicit antibodies,” Prof Ball said.
Dr Al Edwards, from the University of Reading’s school of pharmacy, added: “Those vaccines might be less attractive based on these studies because if the real viral infection doesn't give you a long and powerful immune response, you might expect that by weakening the virus you have an even lower immune response.”
Read more: How far away is a coronavirus vaccine? Latest news on UK and US trials


