Coronavirus vaccine 'for all' unlikely before 2022, says leading figure

The coronavirus vaccine development effort still needs around $800m (£623m) to get the leading candidates "over the line", according to one of its central figures.
Dr Richard Hatchett, chief executive of the Coalition for Epidemic Preparedness Innovations (Cepi), said the sums involved were huge, but paled in comparison to what the coronavirus pandemic costs the world on a daily basis.
In an interview with The Telegraph, he also warned that demand is likely to outstrip supply for a successful vaccine - if one can be produced - until at least 2022, although he expects the first doses to be available from the first half of 2021. But major challenges around scaling up the manufacture and distribution of the vaccine remain, he said.
"This will be the single largest global vaccine delivery programme in world history," he said. "It is an extraordinary challenge."
Separately, scientists from the Royal Society's Delve (Data Evaluation and Learning for Viral Epidemics) warned on Thursday that a vaccine could take at least a year to distribute, involving 20,000 staff members and even field hospitals.
On top of the money needed for research and development for some of the leading vaccine candidates - nine of which are in the final stage of clinical trials - Dr Hatchett said around $5bn (£3.9bn) would be needed for manufacture, some of which has already been pledged.
The global effort to ensure that vaccines are available equitably for lower- and middle-income countries also needs around $4.6bn ( £3.6bn), he said, in order to procure doses.
That effort is part of Covax, an initiative led by Cepi, the World Health Organisation and Gavi, the Vaccine Alliance, to develop, procure and distribute a Covid-19 vaccine. This week it revealed almost $1bn (£770m) in new pledges, including from the World Bank, Great Britain and Canada.
"When you talk about these big numbers you have to put it up against the types of damages we are sustaining," said Dr Hatchett. For example, the International Monetary Fund (IMF) has estimated that the pandemic will wipe $6trn (£4.6trn) off the global economy this year - so the total costs of the vaccine programme could be covered by the losses sustained in just two days. As such, shortening the pandemic by even six months could have an "extraordinary" impact, he said.
"We are still $7-800m dollars short of what we think we need to bring these promising vaccines across the finishing line," he said. In total, research and development will cost $2.1bn (£1.6bn), over half of which has already been raised.
"We've had really remarkable progress...but there is a substantial amount still to be done. We don't know which vaccines are going to work, we don't know how well they are going to work, or how they are going to work in different populations."
He described it as an "extraordinarily busy, exciting, encouraging, frightening" moment.
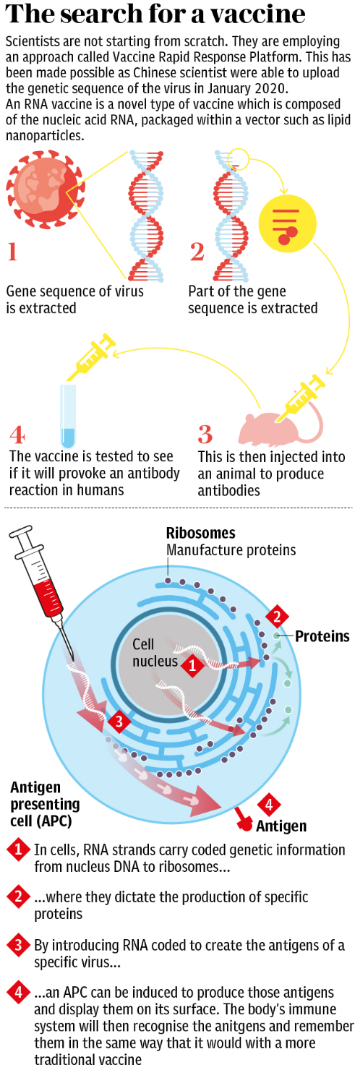
The push for a vaccine is being financed by governments, philanthropic organisations, and private donors, with some of the costs also being borne by vaccine manufacturers.
Some countries, including the United States and China, have not joined Covax, but Dr Hatchett said it was worth remembering that the US has invested up to $12bn (£9.3bn) in developing a number of different vaccines via its Operation Warp Speed programme, more than the rest of the world combined. However, he was unconvinced by the noises coming out of the US about getting a vaccine by November.
"I think Mr Trump is ambitious in thinking we will have a vaccine before the election. It is possible we will have the data before the end of the year to allow the FDA to issue an emergency use license if there is strong evidence of efficacy. But what we won't have at that point is what we would consider to be a mature safety database," he said.
The FDA has indicated it is looking for six months of safety data, and particularly because many of the leading vaccine candidates require two doses, that means there is some way to go even for the jabs that are currently in their final stage of trials. This is why Dr Hatchett doesn't believe a vaccine will be available before next year, a timeframe which he said should reassure people with concerns over their safety, he said.
Moreover, he said the world should be braced for other potential delays - safety issues like the health scare which paused the AstraZeneca trials, but also manufacturing problems.
For example, during the swine flu pandemic in 2009, one vaccine that was safe, effective and licensed simply could not be produced at a large-scale.
"The yields were so low - we thought we were going to have 100m doses of the vaccine but we only had 30m," said Dr Hatchett.
"Vaccine development is really risky - it's not just a matter of it is safe and effective then you're off to the races to manufacture at scale. You can run into speed bumps at many steps."
That includes distribution.
"Some have to be stored at minus 70 degrees celsius, which is still a challenge in many environments," Dr Hatchett said. "This is likely to lead to restricted availability in many countries."
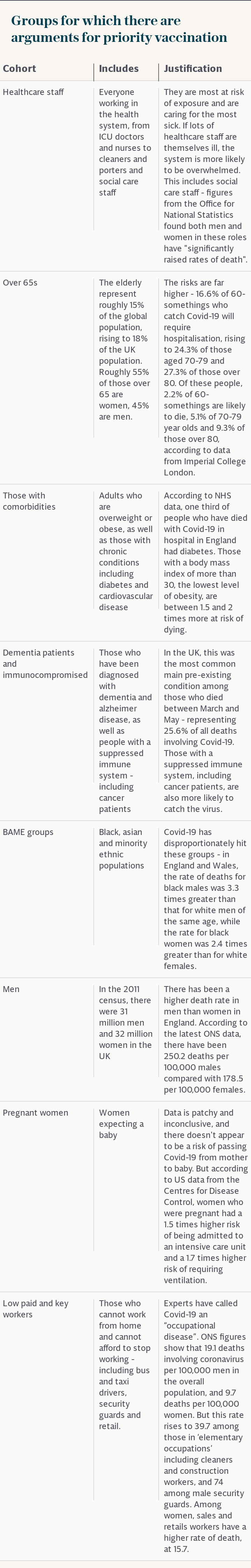
Overall, he said these challenges mean he does not expect vaccine supply to equal demand before 2022 or 2023, so healthcare workers and then those most vulnerable to the virus should be initially prioritised for protection, he said.
"If we can do that, we can convert what is a really scary pandemic into something that seems much more manageable and tolerable," he said. "If we protect those at greatest risk that allows life to begin to come back to normal even before we can bring up supply to meet the demand of everybody who wants to be vaccinated."
He added: "We can take the edge off the pandemic by the end of next year, and we'll see supply come into equilibirum by 2022, certainly by 2023."
And he said that the unprecedented speed of the response to Covid-19 could present a much-needed framework for tackling inevitable future pandemics.
"This is already the second pandemic of the 21st century," he said. "It is certainly not the last."


