Cartesian (RNAC) MG Study Meets Goals, Stock Down on Other Updates
Cartesian Therapeutics, Inc. RNAC announced positive top-line results from its mid-stage study evaluating lead candidate Descartes-08 in patients with generalized myasthenia gravis (MG), an autoimmune disease associated with muscle weakness.
A total of 36 heavily pre-treated, highly symptomatic MG patients were randomized equally to receive either Descartes-08 or placebo, administered as six weekly outpatient infusions without preconditioning chemotherapy in this phase IIb double-blind, placebo-controlled, crossover study. Patients receiving placebo were eligible to cross over to Descartes-08 treatment at the conclusion of the trial’s blinded follow-up assessment after three months.
Descartes-08 is an autologous mRNA-engineered chimeric antigen receptor T-cell therapy (mRNA CAR-T) directed against the B cell maturation antigen.
mRNA CAR-T administration does not require preconditioning chemotherapy, can be administered in the outpatient setting, and does not carry the risk of genomic integration associated with cancerous transformation unlike conventional DNA-based CAR T-cell therapies.
The study achieved its primary endpoint with statistical significance in the pre-specified modified intent-to-treat efficacy population. Results showed 71% of MG patients treated with Descartes-08 had 5-point or greater improvements in MG Composite (MGC) score at month three compared to only 25% treated with placebo.
The study also achieved its primary endpoint with statistical significance in the per-protocol population. 69% of patients treated with Descartes-08 had 5-point or greater improvements in MGC score at month three compared to 33% treated with placebo.
Moreover, Descartes-08 responders experienced deep improvements across the MG severity scales at month three. These improvements persisted or further improved in patients who were evaluated after four and six months follow-up visits, as of the cutoff date of Jun 19, 2024. The results were also consistent with the previously reported results from the phase IIa open-label portion of the trial.
The safety profile of the candidate continues to support outpatient administration.
Concurrently, Cartesian announced positive updated results from two patients enrolled in the phase IIa open-label portion of the trial. Both retreated patients experienced rapid improvement in clinical scores and maintained minimal symptom expression for up to one year after receiving a second treatment cycle.
Cartesian expects to hold an end-of-phase II meeting with the FDA by year-end. The candidate was previously granted Regenerative Medicine Advanced Therapy Designation and Orphan Drug Designation by the FDA for the treatment of MG.
Descartes-08 is also being evaluated for systematic lupus erythematosus (SLE). Cartesian also announced the dosing of the first patient in its mid-stage open-label study evaluating Descartes-08 in patients with SLE.
The phase II open-label study is likely to enroll up to 30 adult patients. The study is designed to evaluate the safety and tolerability of outpatient administration of Descartes-08 without preconditioning chemotherapy for the treatment of patients with moderate or severe SLE refractory to immunosuppressant therapy. Secondary outcome measures will assess overall disease activity.
Shares of Cartesian have lost 23.8% year to date compared with the industry’s decline of 6.3%.
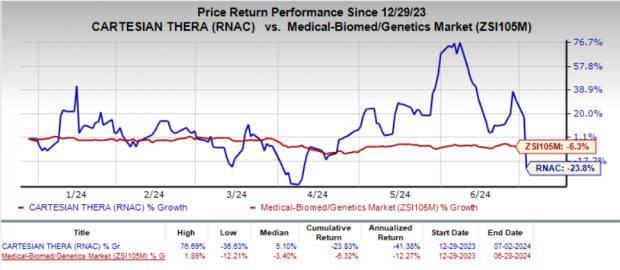
Image Source: Zacks Investment Research
Despite the positive results from the MG study, shares of RNAC plunged 35% on Jul 2.
This can likely be attributed to the company’s announcement of a $130-million private placement equity financing through the sale of shares priced at $20 each.
The net proceeds from this financing, together with the existing cash, cash equivalents, and marketable securities, should enable the company to fund its pipeline programs for Descartes-08 and general corporate purposes and working capital.
Nonetheless, the successful development of Descartes-08 for MG should be a significant boost for the company.
argenx’ ARGX Vyvgart Hytrulo is currently approved in the United States for the treatment of GMG in adult patients who are anti-acetylcholine receptor antibody positive.
ARGX recently won an FDA nod for a second indication for Vyvgart Hytrulo, chronic inflammatory demyelinating polyneuropathy.
Zacks Rank & Stocks to Consider
Cartesian currently carries a Zacks Rank #3 (Hold).
A couple of better-ranked stocks in the biotech sector are ALX Oncology Holdings ALXO and Minerva Neurosciences, Inc. NERV, both carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 30 days, the Zacks Consensus Estimate for ALX Oncology’s 2024 loss per share has narrowed from $3.33 to $2.89. During the same period, the consensus estimate for 2025 loss per share has narrowed from $2.85 to $2.73.
ALX Oncology beat on earnings in two of the trailing four quarters and missed the mark in the other two, delivering an average negative surprise of 8.83%.
In the past 90 days, estimates for Minerva Neurosciences’ 2024 loss per share have narrowed from $3.57 to $1.89. The loss per share estimate for 2025 has narrowed from $4.54 to $3.60.
NERV’s earnings beat estimates in one of the trailing four quarters and missed the same in the other three, the average negative surprise being 54.43%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Minerva Neurosciences, Inc (NERV) : Free Stock Analysis Report
argenex SE (ARGX) : Free Stock Analysis Report
ALX Oncology Holdings Inc. (ALXO) : Free Stock Analysis Report
Cartesian Therapeutics, Inc. (RNAC) : Free Stock Analysis Report

