Annovis Bio (ANVS) Begins Dosing in Phase III Parkinson's Study
Annovis Bio ANVS announced the dosing of the first patient in a phase III study evaluating the company’s lead candidate, buntanetap, for the treatment of Parkinson’s Disease (PD).
The company announced top-line data from its phase IIa study of buntanetap in PD patients, treated with multiple-dose cohorts (5mg, 10mg, 20mg, 40mg or 80mg) once daily, back in October 2021. The study found buntanetap is safe, exhibiting no adverse effects in the subjects.
Moreover, compared to the placebo-controlled group, in the phase IIa study, buntanetap demonstrated statistically significant improvements in motor dexterity, speed and accuracy in early-to-moderate PD patients.
Post the success of the phase II study, the company had meetings with the FDA to transition the drug to its late-stage trial.
Last month Annovis received a positive notice from the FDA to progress with the phase III study of buntanetap in PD.
The randomized, double-blind, placebo-controlled late-stage study evaluates the safety, efficacy and tolerability of 20mg dosage of buntanetap in 450 PD patients, for six months, on top of their standard care procedure.
Apart from PD, buntanetap, a translational inhibitor of neurotoxic proteins is also being evaluated in Alzheimer’s disease (AD) and dementia in Down syndrome (AD-DS).
Stock price of Annovis Bio’s was up 5.88% during market hours on Aug 24, post the announcement of the aforementioned news. Annovis shares have lost 35.5% in the year-to-date period compared with the industry’s 21.6% decline.
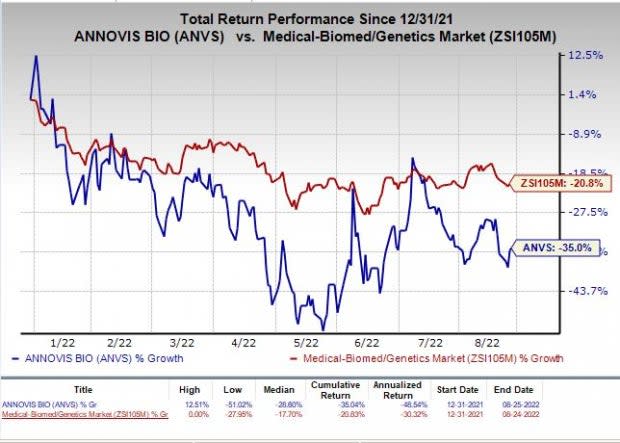
Image Source: Zacks Investment Research
The target market has potential. In May, Pharma giant AbbVie ABBV submitted a new drug application to the FDA for ABBV-951 (foscarbidopa/foslevodopa) for treating motor fluctuations in patients with advanced PD.
AbbVie’s ABBV-951 has been designed to offer the first continuous subcutaneous delivery of carbidopa/levodopa (CD/LD) prodrugs for PD patients.
Annovis Bio, Inc. Price
Annovis Bio, Inc. price | Annovis Bio, Inc. Quote
Zacks Rank and Stocks to Consider
Annovis Bio currently has a Zacks Rank #3 (Hold).
Some better-ranked stocks in the same sector are Rhythm Pharmaceuticals RYTM and Pliant Therapeutics PLRX, each carrying a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 (Strong Buy) Rank stocks here.
Rhythm’s loss per share estimates for 2022 have widened from $3.74 to $3.82 in the past 30 days. The same for 2023 has narrowed from $3.40 to $3.08 in the same time frame.
Earnings of Rhythm missed estimates in two of the trailing four quarters and beat the same on the remaining two occasions. The average earnings surprise for RYTM is 2.97%.
Pliant’s loss per share estimates for 2022 have improved from $3.28 to $3.01 in the past 30 days. The same for 2023 has narrowed from $3.68 to $3.29 in the same time frame.
Earnings of Pliant beat estimates in two of the trailing four quarters, was in one, and missed estimates in remaining one occasion. The average earnings surprise for PLRX is 0.90%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AbbVie Inc. (ABBV) : Free Stock Analysis Report
Rhythm Pharmaceuticals, Inc. (RYTM) : Free Stock Analysis Report
Annovis Bio, Inc. (ANVS) : Free Stock Analysis Report
Pliant Therapeutics, Inc. (PLRX) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

